In the latest edition of our PackReview video series, DDL Inc. Package Engineers Scott Levy and Pete Johnson continue their discussion on ASTM F88 Seal Strength. In this video, Scott and Pete address the one-pound minimum myth, design validation, specification of seal strength and some upcoming changes on the horizon with ISO 11607.
Seal Strength Acceptance Criteria
Currently, most customers use a one-pound minimum seal strength requirement within their protocols. Because of this, DDL often sees customers failing their protocols because they are not meeting this one-pound acceptance criteria. According to Scott, almost every protocol he has seen has had a one-pound minimum seal strength requirement.
Where did this one-pound minimum come from? Based on his research, EN868 has a strength requirement, and going through all of the calculations it gets close to one pound. “However, that one pound does not have much meaning to me,” Scott says. “One-pound or minimum strength requirements should be coming directly from your process validation. Before determining what type of strength that is needed, look at the type of product you have that is going into a particular pouch or tray.”
Design Validation
In design validation, you need to take a look at the strength levels says Scott. “For example, would you put a ten-pound bowling ball inside a pouch with a one-pound minimum strength requirement? Of course not. But, if you put gauze inside a pouch, is one pound an acceptable strength? Absolutely.” Each medical device manufacturer needs to decide on what strength level is acceptable. The key aspect is being able to have repeatability and reproducibility of that strength level you are claiming is your minimal strength. The one-pound minimum has manifested itself as the industry norm, but the one-pound minimum may not be best for your particular application.

Seal Strength Specifications
According to Scott, you should have acceptance criteria to fully understand where you are at with your seal strength testing. Again, the key is consistency in reproducibility and repeatability. In package validation, you want to ensure you have high confidence the strength levels you have been producing after aging and distribution are either equal or better than what you are stating is your strength requirement. Many are doing their strength testing only pre-sterilization. In Scott’s opinion, you should be taking a look at your seal strength levels both pre-sterilization and post-sterilization, as well as after transportation distribution testing, to better understand if sterilization is going to cause any adverse effects.
ISO 11607 Updates on Usability
A big change medical device manufacturers are going to have to deal soon will be the new ISO 11607 revision on usability. During the design validation you are going to have to go out and speak with the end user (scrub nurse, OR nurse, home user etc.) to understand the overall usability of a package. At that, point understanding the strength levels for a specific nurse to open that package is going to be important information.
Package engineers have had to deal with usability of products for years. Now with ISO 11607 being updated, packaging engineers are going to also have to deal with usability of package materials as well (ease of opening, ease of aseptic transfer etc.), and this will be new to many people.
For more information on F88 Seal Strength testing, please contact us or call us at 800-229-4235.
 DDL once again was a sponsor and attendee of the annual
DDL once again was a sponsor and attendee of the annual  DDL announced it has relocated its California lab from Fountain Valley to a facility located in Irvine. The new facility more than doubles the lab space, which will increase DDL’s package testing capacity and ability to grow to better meet its customer’s testing needs.
DDL announced it has relocated its California lab from Fountain Valley to a facility located in Irvine. The new facility more than doubles the lab space, which will increase DDL’s package testing capacity and ability to grow to better meet its customer’s testing needs. Other sessions at HealthPack included:
Other sessions at HealthPack included: In addition to many informative sessions, a great part about attending conferences such as HealthPack and ISTA TransPack Forum is it provides an opportunity for members of the medical device packaging industry to reconnect with old colleagues, as well as interact with customers and meet new contacts.DDL looks forward to participating in next year’s HealthPack being held March 6-8, 2018 in Kansas City, MO, and the ISTA Transpack Forum being held March 20-23, 2018 in San Diego, CA.
In addition to many informative sessions, a great part about attending conferences such as HealthPack and ISTA TransPack Forum is it provides an opportunity for members of the medical device packaging industry to reconnect with old colleagues, as well as interact with customers and meet new contacts.DDL looks forward to participating in next year’s HealthPack being held March 6-8, 2018 in Kansas City, MO, and the ISTA Transpack Forum being held March 20-23, 2018 in San Diego, CA. ASTM D4169
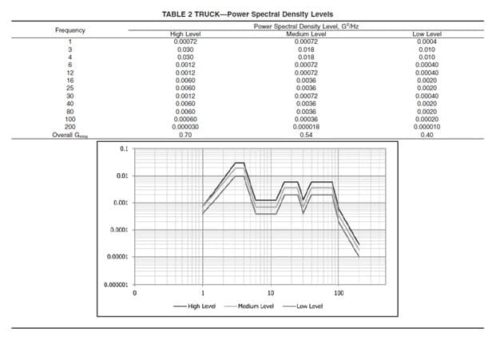
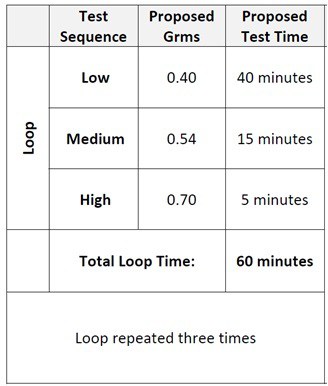
ASTM D4169